FACSAriaIIu Cell Sorter

BD FACSAriaIIu, 4-laser, 9 colour
| 488nm Blue Laser | ||
| Alexa Flouor 488, FITC, BB515 | 530/30 | 502LP |
| PE | 575/26 | 556LP |
| PE-Texas Red | 610/20 | 595LP |
| PI, PerCP, PerCP-Cy5.5, BB700 | 695/40 | 655LP |
| PE-Cy7 | 780/60 | 735LP |
| 633nm Red Laser | ||
| APC, Alexa Fluor 647 | 660/20 | N/A |
| APC-Cy7, APC-H7 | 780/60 | 735LP |
| 375nm Near UV Laser (choose one) | ||
| DAPI/Hoechst Blue (Cell cycle) | 450/40 | N/A |
| Hoechst Red (Side population) | 670LP | 610LP |
| 405nm Violet Laser (choose one) | ||
| Alexa Fluor 405, V450, BV421, Pacific Blue | 450/40 | N/A |
| Alexa Flouor 430, AmCyan, V500, BV510 | 510/50 | 502LP |
Consumables
(1) Nozzle : 70, 85 and 100 um
(2) Collection devices :
Two way-, Four way sorting: 5mL Falcon tubes or 15 mL tubes
Plate sorting: 6-, 12-, 24-, 48- and 96-well plate
(3) Sample tube : 5mL Falcon tubes or 15 mL tubes
1. Sample preparation
(1) Recommend sample concentration
|
Nozzle |
Cell type |
Concentration |
|
70 μm |
Lymphocytes, thymocytes or splenocytes (diameter 8-12μm) |
8~12 x 106/ml |
|
85 μm |
Activated lymphocytes, small cell lines (diameter 12-20μm) |
7~9 x 106/ml |
|
100 μm |
Large adherent cell line (diameter >20μm) |
5~8 x 106/ml |
(2) Filter your samples
Recommend filtering samples through a nylon mesh 40 µm (BD Cat. No. 352340) or 5-mL polystyrene test tubes, 12 x 75-mm (BD Falcon) with cell-strainer cap (BD Cat. No. 352235) if aggregated cells can be seen in the sample tube.
Filtered sample should be saved on ice, protected from light.
Q&A
Q1. What kind of buffer solution is better for sample preparation? Can be suspended in culture medium?
Ans. Sample is not recommend to suspend the cells in culture medium because Phenol Red in medium will interfere with the fluorescence signal.
§ Basic Cell Sorting Buffer:
1x PBS (Ca/Mg++ free) or HBSS (preferred)
0.5-2% BSA or up to 2% heat-inactivated FBS [dialyzed against Ca/Mg free PBS]
25mM HEPES pH 7.0
§ For Clean Lymphoid Cells: The buffer can be simplified to HBSS with 1% FBS. The additional cations in the recipe promote better viability. Since these cells are not prone to clump, the lack of EDTA is not a problem.
§ For Sticky Cells: Raise the concentration of the EDTA to 5mM and use FBS that has been dialyzed against Ca/Mg++ free PBS. Some activated cells become clumpy and the chelators (EDTA) help reduce cation-dependent cell to cell adhesion.
§ For Adherent Cells: In order achieve good single cell preparations, one must start at the moment of detaching your cells from the plate. Typically, the trypsin (or other detachment buffer) is quenched with culture media or a PBS/FBS buffer. This is problematic because it reintroduces the cations that facilitate the cells reattaching to the plate (or each other). One must use a cation-free FBS buffer in order to stop the detachment. Additionally, the level of EDTA can be increased if necessary (but too much EDTA can be deleterious).
§ For Samples with High Percentage of Dead Cells: If there are a large number of dead cells in the prep, it is likely that there is soluble DNA from the dead cells that will come out of solution. This DNA will start to coat the cells and and lead to severe clumping. The addition of 10U/mL DNAase II to the buffer recipe will help reduce DNA associated clumpiness.
Q2. Should the collection tubes be filled with buffer?
Ans: All collection tubes should be pre-coated with 1% BSA or 10% FBS in 4℃, overnight. It would avoid sorted cells sticking to the sides of the tubes. Collection tubes should be filled 1/3 or 1/2 full with the appropriate collection media.
§ 15mL conical should be filled to 7~8mL with the culture medium。
§ 5mL Falcon tube should be filled to 2~3mL with the culture medium。
Q3. Would sorted cells be contaminated during sorting process?
Ans: The instrument is not in aseptic environment so that it is recommend to add antibiotic to avoid contamination. Wash sorted cells once or twice with culture medium is recommend.
Data
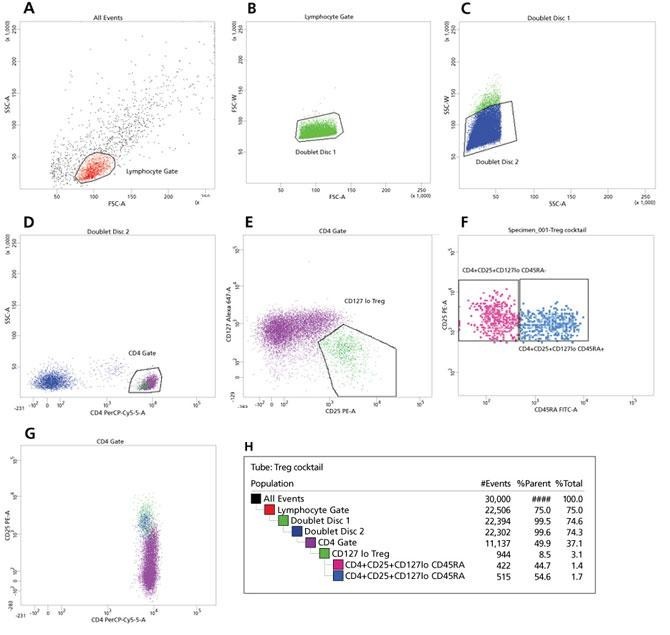
Gating strategy used to sort CD45RA+ and CD45RA- Tregs
The BD FACSAriaII system was set up for a sort using either a 70-μm or 100-μm nozzle (70 psi or 35 psi with a frequency of 87 or 60 kHz respectively). CD45RA+ Tregs and CD45RA- Tregs were sorted in purity mode at a rate of 10,000 to 11,000 events per second.
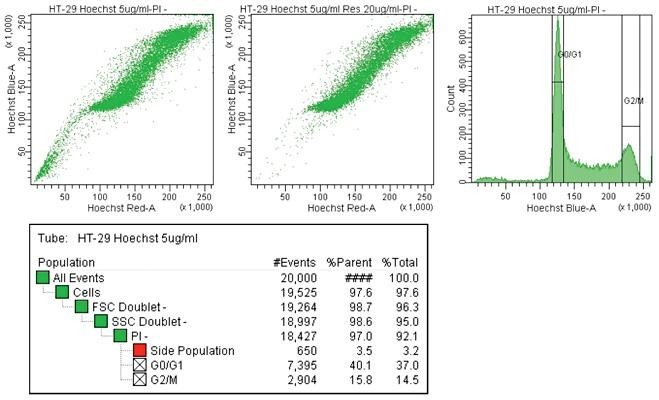
Cancer cell line side population
Human HT-29 colon cancer cells were stained with Hoechst 33342 and acquired on the BD FACSAria III equipped with a 375-nm laser (left dot plot). As a control, side population expression was blocked (right dot plot).
Attachment:
2. FACSDiva8_FACSAria_quickguide
BD Bioscience http://www.bdbiosciences.com/tw/instruments/facscalibur/features/applications.jsp