流式細胞分選儀 FACSAriaIIu Cell Sorter
流式細胞分選儀 FACSAriaIIu Cell Sorter

BD FACSAriaIIu, 4-laser, 9 colour
| 488nm Blue Laser | ||
| Alexa Flouor 488, FITC, BB515 | 530/30 | 502LP |
| PE | 575/26 | 556LP |
| PE-Texas Red | 610/20 | 595LP |
| PI, PerCP, PerCP-Cy5.5, BB700 | 695/40 | 655LP |
| PE-Cy7 | 780/60 | 735LP |
| 633nm Red Laser | ||
| APC, Alexa Fluor 647 | 660/20 | N/A |
| APC-Cy7, APC-H7 | 780/60 | 735LP |
| 375nm Near UV Laser (choose one) | ||
| DAPI/Hoechst Blue (Cell cycle) | 450/40 | N/A |
| Hoechst Red (Side population) | 670LP | 610LP |
| 405nm Violet Laser (choose one) | ||
| Alexa Fluor 405, V450, BV421, Pacific Blue | 450/40 | N/A |
| Alexa Flouor 430, AmCyan, V500, BV510 | 510/50 | 502LP |
相關配備
(1) 噴嘴 (Nozzle) : 70, 85 and 100 um
(2) 收集管 (Collection devices) :
兩向分選、四向分選: 5mL Falcon tubes or 15 mL tubes
盤式分選: 6-, 12-, 24-, 48- and 96-well plate
(3) 上樣管 (Sample tube) : 5mL Falcon tubes or 15 mL tubes
1. 檢體置備之注意事項
(1) 建議樣本濃度
|
Nozzle |
Cell type |
Concentration |
|
70 μm |
Lymphocytes, thymocytes or splenocytes (直徑8-12μm) |
8~12 x 106/ml |
|
85 μm |
Activated lymphocytes, small cell lines (直徑12-20μm) |
7~9 x 106/ml |
|
100 μm |
Large adherent cell line (直徑>20μm) |
5~8 x 106/ml |
(2) 檢體上樣前過篩
檢體上樣前,為了降低阻塞物的可能,細胞樣本在分選之前需要用40 μm的篩網過濾(BD Cat. No. 352340) 或含篩網蓋試管(BD Cat. No. 352235),過濾後細胞應是繼續避光冰浴保存。容易黏附的細胞,則有必要考慮在即將分選前再進行篩網過濾,因為冰存時間一長,細胞可能再度聚集。
Q&A
Q1. 上樣之檢體應置配在何種緩衝液裡? 可以直接懸浮在培養液裡嗎?
Ans. 不建議將細胞懸浮於medium中,因為medium中的 Phenol Red 會干擾螢光訊號,影響分選的結果,請於上機前將細胞懸浮於樣品緩衝液中。
§ 建議的基礎配方:
1x Phosphate Buffered Saline (不含鈣、鎂離子)
1mM EDTA
25mM HEPES pH 7.0
1% Fetal Bovine Serum (Heat-Inactivated)
0.2um filter sterilize, store at 4℃
§ 淋巴系細胞樣本緩衝液配方:
1x HBSS with 1% FBS
HBSS配方中的陽離子可增進細胞的生存力。由於這些細胞並非易於叢集細胞,配方中缺乏EDTA不成問題。
§ 為黏附細胞設計的配方:
Raise the concentration of the EDTA to 5mM and use FBS that has been dialyzed against Ca/Mg++ free PBS。EDTA可抑制細胞黏附。
§ 為貼壁型細胞株設計的配方:
為了完成單細胞懸浮液的準備,必須從detach步驟就開始注意。一般先以 PBS 潤洗,再以Trypsin處理以釋放細胞,待細胞變圓(切記不可過度作用),便以 5 ml 含5%血清培養液收取細胞,並均勻地打散細胞懸浮液。調整細胞濃度,並換成基礎樣本緩衝液配方。
§ 為含有高比例死細胞的樣本:
基礎配方加5mM MgCl2加25~50 ug/mL DNAase I (Sigma D-4513)。這緩衝液配方將幫助減少因DNA造成的細胞聚集。
這些建議將有助於在處理樣本時,提高整個細胞族群生存能力,並在後續分選程序中提昇標靶細胞的回收率。
Q2. 分選收集管裡面需要置放buffer嗎?
Ans: 分選收集管可以事先加以coating, 提高細胞存活率 (1% BSA或 10% FBS, 4℃, overnight) 。
§ 以 15mL 離心管收集細胞時, 一般建議先於管內加入 7~8mL 收集液 (medium, PBS或其他溶液)。
§ 以 5mL Falcon tube 收集細胞時, 一般建議先於管內加入 2~3mL 收集液。
Q3. 如果分選出來的樣品要在培養,會汙染嗎?
Ans: 儀器在開關機時會進行清洗,但因為儀器所處環境仍非無菌,故通常建議帶來要接收分選產物的Meduim要加入抗生素,以減低污染之機率。
Data
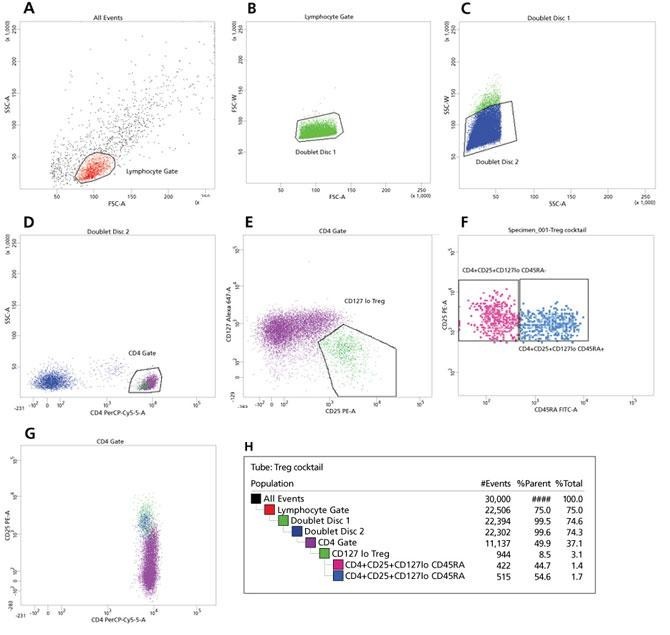
Gating strategy used to sort CD45RA+ and CD45RA- Tregs
The BD FACSAriaII system was set up for a sort using either a 70-μm or 100-μm nozzle (70 psi or 35 psi with a frequency of 87 or 60 kHz respectively). CD45RA+ Tregs and CD45RA- Tregs were sorted in purity mode at a rate of 10,000 to 11,000 events per second.
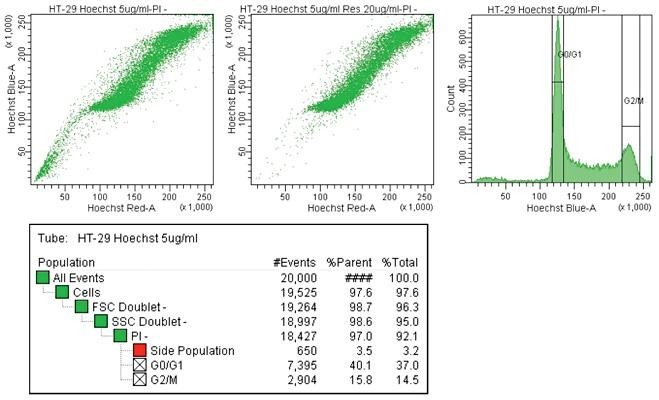
Cancer cell line side population
Human HT-29 colon cancer cells were stained with Hoechst 33342 and acquired on the BD FACSAriaIII equipped with a 375-nm laser (left dot plot). As a control, side population expression was blocked (right dot plot).